Glutathione Injection Side Effects: Risks, Evidence, What Doctors Want You to Know
In This Article
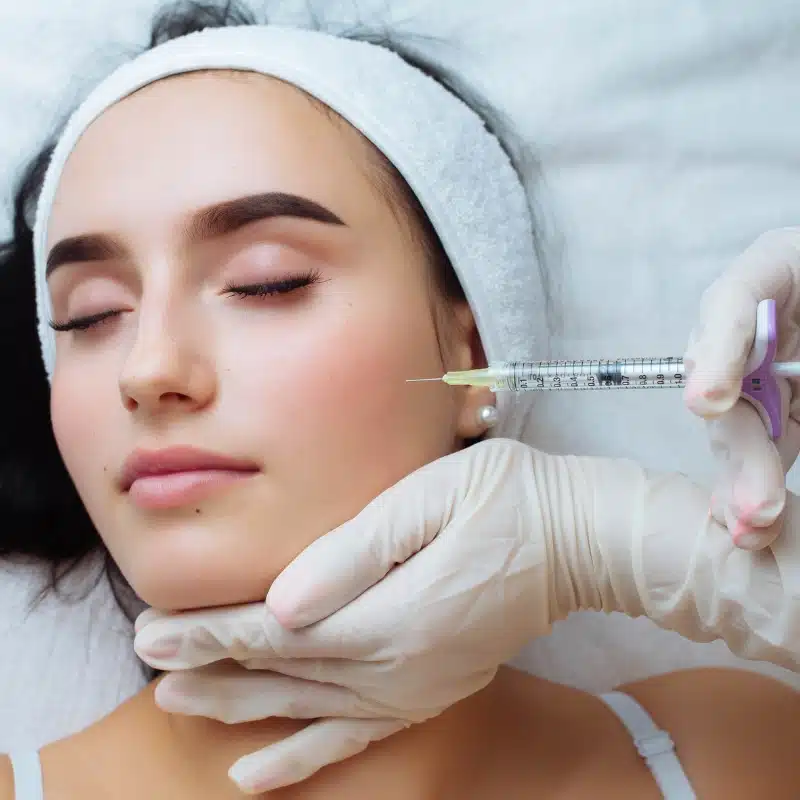
Highlights
- Intravenous (IV) or injection-based glutathione is not approved in most countries for skin-whitening or cosmetic use. (dpcj.org)
- There is limited scientific evidence that injections deliver lasting skin-lightening benefit — and what evidence exists suggests effects may fade after treatment stops.
- Side-effects range from mild (nausea, injection-site discomfort) to serious and even life-threatening (allergic reactions, liver injury, systemic inflammatory response, endotoxin-related events). (PubMed)
- Safety depends heavily on product purity, sterile technique, dosing, and supervision by licensed medical professionals. Unregulated or poorly compounded injections carry higher risks. (U.S. Food and Drug Administration)
- If you’re considering glutathione for skin-lightening or wellness, oral or topical alternatives appear safer, although their effects are more modest. (PubMed)
Bottom line: Glutathione injections are risky. Clinics offering them purely for cosmetic lightening should be approached with caution — and discussed thoroughly with a qualified doctor.
What Is Glutathione & Why Do Some People Use Injections?
Glutathione is a naturally occurring antioxidant produced in our cells. It helps neutralize free radicals, supports detoxification, and contributes to various metabolic processes. Because of these properties, glutathione has been studied and used medically (in controlled settings) — for example, as a supportive antioxidant therapy in certain chronic diseases or alongside chemotherapy. (PubMed)
In recent years, especially in cosmetic and wellness markets, many clinics have begun offering intravenous (IV) or injection-based glutathione — often marketed as skin-lightening or “brightening,” anti-aging, or detox-boosting treatments. The idea is that injecting glutathione gives higher, more immediate systemic levels than oral or topical forms, which theoretically could affect skin pigmentation more strongly. (PubMed)
However, while some small studies report mild pigment/lightening changes with glutathione (oral or topical), the clinical evidence for long-term, safe, and effective skin-whitening via IV glutathione is weak or absent. (PubMed)
Moreover, many regulatory agencies and dermatology bodies have expressed concern or even warned against the unregulated use of glutathione injections for cosmetic purposes, because of safety, contamination, dosing, and lack of evidence.
Key Differences: Injection Glutathione vs Oral or Topical — Why Risks Increase
| Form of Glutathione | How It Works / Delivered | Relative Safety & Evidence |
| IV / Injectable (IV or IM) | Direct bloodstream delivery → rapid and high systemic levels | Higher risk: needs sterile compounding, strict dosing, sterile technique; little long-term safety data and many unapproved uses. (PubMed) |
| Oral (capsules, tablets, sublingual) | Absorbed via GI tract → slower, lower systemic levels | Better safety record; mild to moderate side effects; relatively better studied. (PubMed) |
| Topical (creams, lotions, soaps) | Local effect on skin surface / upper layers | Safest among the three; limited to modest pigment changes; risks are minimal if formulation is proper. (PubMed) |
Because injection bypasses natural barriers and goes straight into the bloodstream, any impurity, contamination, or incorrect dosing/administration can cause serious harm — unlike oral or topical use which are inherently less invasive. This is a major reason experts urge caution with IV glutathione, especially when used purely for cosmetic skin-lightening. (dpcj.org)
Common & Mild Side Effects (Short-Term) — What People Often Experience
When glutathione is injected, some people report relatively mild — but discomforting — reactions. These may not be dangerous, but they matter.
- Nausea, queasiness, or vomiting — because the body suddenly gets a high antioxidant load.
- Headache or light-headedness — especially if infusion is administered too fast or hydration is poor.
- Injection-site pain, redness, swelling or itching — common to many injections; varies with administration technique.
- Mild allergic reactions (rash, itching, mild swelling) — sometimes seen even with proper technique.
- Transient breathing discomfort or mild bronchospasm — reported in some individuals, particularly those with asthma or sensitive airways.
Because these side-effects are often mild and temporary, they are sometimes dismissed. But repeated sessions — especially at high doses — can increase cumulative risk.
Serious & Rare — But Documented — Adverse Effects
This is where the biggest risks lie. Several medical reports, regulatory advisories, and reviews have documented severe, potentially life-threatening reactions tied to injectable glutathione — especially when done in unregulated settings or with contaminated/inappropriately compounded products.
-
Systemic Inflammatory Response – Shock-like Reaction
A recent (2025) case report described a patient who developed full-blown systemic inflammatory response syndrome (SIRS) after a high-dose, unregulated glutathione infusion — for cosmetic skin-lightening. She had fever > 41 °C, very high inflammatory markers, acute liver injury and clotting abnormalities. No infection source was identified; doctors suspected endotoxin contamination or supraphysiological glutathione dose in a nutritionally compromised host. The patient recovered with supportive care. (PubMed)
This shows that IV glutathione — especially from unregulated sources — can trigger life-threatening inflammation.
-
Contamination & Endotoxin Risk — Severe Adverse Events
In 2019, the regulatory agency in the United States (U.S. Food and Drug Administration / FDA) issued a safety alert after receiving reports of multiple patients who suffered adverse events shortly after IV glutathione that was compounded using dietary-supplement grade powder — an inappropriate ingredient for sterile injectables. Reactions included nausea, vomiting, chills, low blood pressure, difficulty breathing, and in some cases, possible bloodstream infection. Tests confirmed excessive bacterial endotoxin levels in the compounded powder — up to five times safe limit. (U.S. Food and Drug Administration)
This highlights a critical risk: many glutathione “injections” are not pharmaceutical-grade and may not meet sterility standards — making contamination and serious immune reactions possible.
-
Liver & Kidney Stress, Organ Toxicity
A controlled clinical study (treatment group: 1,200 mg IV twice a week for six weeks) comparing glutathione vs placebo found that 8 out of 16 participants developed deranged liver function tests. One patient even had anaphylaxis. Over time, the skin-lightening effect faded: by 6-month follow-up, only 1 out of 16 maintained visible benefit.
A broader narrative review published in 2024 also concluded that IV glutathione is associated with serious safety concerns — including anaphylaxis and hepatotoxicity, and stressed that there is insufficient evidence to recommend IV use for cosmetic skin lightening. (PubMed)
-
Other Rare but Reported Risks
-
- Severe skin reactions — including serious allergic or hypersensitivity reactions. Some reports in literature and reviews list risks like possible skin-peeling disorders (rare). (Science-Based Medicine)
- Potential thyroid, kidney or metabolic disturbances — some anecdotal reports and reviews raise these as possible but under-studied risks. (Science-Based Medicine)
- Cardiovascular effects — rapid high-dose IV injections may cause vasodilation or sudden drop in blood pressure; in rare cases, irregular heart rhythms, dizziness, fainting or collapse — especially in people with pre-existing heart or electrolyte problems.
Because of these risks, many dermatologists and medical bodies strongly discourage the use of injectable glutathione for cosmetic/lightening purposes.
What Does the Scientific Evidence Say — Efficacy & Safety (2025 Overview)
When we examine the medical literature (clinical studies, reviews, regulatory data), a clearer — and more cautionary — picture emerges:
- A 2015 review of all published studies up to that time concluded that: there is no robust evidence supporting IV glutathione for skin lightening; long-term safety data is absent. (PubMed)
- A 2024 review focused on skin-lightening therapies concluded that glutathione tablets or topical glutathione may offer modest pigment/lightening benefits. But for IV glutathione, lack of efficacy and safety concerns mean it is contraindicated for cosmetic use. (PubMed)
- The same review warns that systemic administration (IV) may shift melanin production, altering skin’s natural photoprotection and potentially increasing the risk of UV-induced skin damage or cancer — a risk especially relevant in sunny climates. (PubMed)
- Regulatory reports: the FDA (USA) highlighted multiple adverse events linked to compounded glutathione injections — from mild to severe and life-threatening. (U.S. Food and Drug Administration)
In short: the medical evidence does not support injecting glutathione for skin-lightening — and there is significant evidence of harm or risk.
Why Risk Varies — Factors That Increase Danger with Glutathione Injections
Several variables influence how risky a glutathione injection may be:
- Quality of the glutathione product: pharmaceutical-grade vs dietary-supplement grade vs repackaged supplements. Supplements / powders sold for oral use are often inappropriately used for injection — and may carry bacterial/ endotoxin contamination. (U.S. Food and Drug Administration)
- Sterility & compounding standards: injections must be prepared under strict sterile conditions. Poor sterile technique can lead to infections, endotoxin reactions, sepsis. (U.S. Food and Drug Administration)
- Dosage and infusion rate: high doses or rapid infusion increase risk of systemic reactions (allergy, shock, organ stress).
- Patient’s health status: people with asthma, compromised immunity, liver/kidney issues, poor nutrition may be more vulnerable. (PubMed)
- Lack of regulatory oversight: Because intravenous glutathione for cosmetic use is generally unapproved, many treatments are unregulated or done in non-medical settings — increasing danger. (dpcj.org)
Who Should Definitely Avoid Glutathione Injections
Glutathione injections pose high risk especially for:
- People with asthma or breathing problems — risk of bronchospasm or allergic reactions.
- People with liver or kidney disease, or a history of organ dysfunction.
- People with immune compromise, chronic illness, or malnutrition.
- Pregnant or breastfeeding women — there is no reliable safety data for these groups.
- People who plan to get frequent injections over long periods — long-term safety is unknown.
- Individuals who would obtain injections from non-medical providers, unlicensed clinics, or via online “cosmetic drip” services.
If you fall into any of these categories — injections are strongly discouraged; safer alternatives (oral/topical glutathione, dermatologic/lightening creams or treatments) are preferable.
If You Experience Side Effects — What You Should Do
- For mild effects (nausea, mild injection-site discomfort, mild rash): stop further injections, hydrate, and monitor symptoms. Consult the provider or a doctor for guidance.
- For serious symptoms (difficulty breathing, swelling, high fever, severe rash/blisters, jaundice, abdominal pain, dark urine, fainting): seek emergency medical care immediately. Bring information about the product (batch number, clinic name, timing).
- Report adverse events to the national drug safety authority in your country (e.g. drug regulatory agency), especially if you suspect contamination or unexpected severe reaction.
If using injections for cosmetic purposes: reconsider — share this article with your clinician and weigh benefits vs risks carefully.
Alternatives to Injectable Glutathione — Safer, Lower-Risk Options
If you are seeking skin brightening / pigment reduction / “glow,” consider:
- Oral glutathione supplements — slower, more modest effects; better safety record. (PubMed)
- Topical glutathione (creams / lotions / soaps) — acts locally on the skin, minimal systemic absorption, lower risk. (PubMed)
- Dermatologist-recommended skin-lightening treatments (chemical peels, laser toning, topical agents) — these have established safety and efficacy profiles (when done properly) compared to unproven injections.
- Healthy skin care routines: sun protection, good moisturization, antioxidants via diet, adequate sleep — natural ways to improve skin health without risking invasive procedures.
Regulatory & Medical Community Viewpoint (2025)
- The leading dermatology and pharmacology reviews caution against parenteral (IV/IM) glutathione for cosmetic skin-lightening — citing lack of robust evidence and significant safety concerns. (PubMed)
- The FDA (USA) explicitly warned compounders about using dietary-supplement-grade glutathione to make sterile injectables, after documented adverse events linked to endotoxin contamination. (U.S. Food and Drug Administration)
- Experts emphasize that injectable glutathione should only be used in medical settings, for approved indications, and never for cosmetic skin-lightening or “beauty treatments.”
As of 2025, many dermatology associations around the world continue to advise against the cosmetic use of IV glutathione.
| Treatment | Best For | Safety |
| Glutathione Injection | Quick brightening, detox | Moderate to high risk |
| Chemical Peels | Pigmentation, glow | Safe when done by dermatologist |
| Laser Toning | Deep pigmentation, melasma | Very effective & safe |
| Oral Glutathione | Gradual results | Safer option |
| Topical Serums (Vit C/ Kojic) | Surface pigmentation | Very safe |
Conclusion — Is It Worth the Risk?
Injectable glutathione may sound appealing as a “quick fix” for skin lightening or general glow — but scientific evidence does not support its effectiveness, and risks are real and significant. From case reports of severe inflammation and liver injury, to regulatory alerts about contamination — the hazards outweigh the unproven benefits.
If you are considering glutathione, oral or topical forms are safer; or better yet, consult a board-certified dermatologist about proven alternatives.
Beauty and skin health should never come at the cost of your systemic health and long-term wellbeing.
Frequently Asked Questions
Mild side effects include nausea, headache, lightheadedness, and injection-site pain/redness. These usually resolve within hours to a few days. (PubMed)
Yes — allergic reactions from mild rash/itching to severe anaphylaxis have been reported; severe allergy is uncommon but possible. (JPAD)
No — injectable glutathione is not approved for cosmetic skin-lightening and the FDA has warned about safety concerns when non-pharmaceutical powders are used for compounding injectables. (U.S. Food and Drug Administration)
There are case reports and small studies showing deranged liver function tests and possible renal stress after IV glutathione; repeated high doses may increase risk. (JPAD)
Unregulated clinics may use non-pharmaceutical powders, poor compounding techniques, or non-sterile equipment — raising the risk of endotoxin contamination, infection and severe immune reactions. (U.S. Food and Drug Administration)
IV produces higher immediate blood levels and may act faster, but evidence shows limited sustained benefit and higher safety risk compared with oral or topical forms. Reviews favor oral/topical for safety. (PubMed)
Avoid if pregnant/breastfeeding, have liver/kidney disease, asthma or severe allergies, immune compromise, or if you cannot confirm product sterility and source. Consult a dermatologist first.
Seek emergency care if you have difficulty breathing, throat/face swelling, fainting, high fever, intense abdominal pain, dark urine, or yellowing of skin/eyes. These can signal anaphylaxis, liver injury, or severe infection. (PubMed)
Stop further injections and report the event to the clinic and your national drug safety authority (e.g., FDA in the US, local health regulator). Keep product information (brand, batch) and date/time of administration. (U.S. Food and Drug Administration)
Long-term data are limited; some experts warn that altering melanin production could reduce natural photoprotection and theoretically increase UV damage risk — more research is needed. (Wiley Online Library)
Yes — topical glutathione, oral supplements, dermatologist-supervised topical agents, chemical peels, and laser therapies have clearer safety profiles and evidence when used appropriately. (PubMed)
There is no internationally accepted safe dose or frequency for cosmetic IV glutathione. Any dosing should only be decided by a licensed clinician and limited to approved medical indications. (dpcj.org)
Yes — reported severe adverse events (e.g., SJS, SIRS) have occurred after combined IV formulations; multi-ingredient drips complicate safety and increase risk of interactions or hypersensitivity. (PubMed)
If past injections were from a licensed medical facility using pharmaceutical-grade product and you have no symptoms, discuss long-term plans with your doctor. If product origin or sterility is unknown, stop and consult a dermatologist.
Our certified subject matter experts do extensive research and collate facts from reputed scientific journals and international studies to create informative and engaging articles related to all your dermatology concerns. They strive to help you decipher medical jargon, distinguish fact from fiction and overcome paranoia. Our qualified medical board or expert panel goes a step further to verify these facts based on their rich academic knowledge, vast clinical experience and critical industry insights to ensure you consume only medically accurate content that empowers you to make informed decisions about your hair and skin-care treatments and weight management. Check out our Editorial policy for further details.
| Year / Source | Finding / Note |
| 2015 — “Intravenous glutathione for skin lightening: Inadequate safety data” (PubMed review) | No long-term safety data for IV glutathione; limited trials; possible increased skin cancer risk due to melanin shifts. (PubMed) |
| 2024 — Systematic review on glutathione in melasma & skin-lightening | Oral/topical glutathione showed modest effect; IV glutathione considered contraindicated due to lack of efficacy and safety. (PubMed) |
| 2025 — Case report: Systemic inflammatory response syndrome (SIRS) after high-dose unregulated glutathione infusion | Patient had shock, fever, acute liver injury — recovered after care; highlights risk of unregulated cosmetic IV use. (PubMed) |
| 2019 — Regulatory alert by U.S. FDA on compounded glutathione injectables contaminated with endotoxin | Several patients had severe reactions; confirms contamination & purity risk. (U.S. Food and Drug Administration) |
| 2022 — Placebo-controlled study (IV glutathione vs saline) over 6 weeks | Minimal efficacy: after 12 injections, only 37.5% had improvement; at 6-month follow-up, effect mostly lost; many had liver function derangement, one had anaphylaxis. (JPAD) |
Read This Next
Laser Toning Treatment: Benefits, Side Effects, Results & Cost In India

Q Switch Laser Treatment In Kolkata: Cost, Results & Procedure

Top 10 Fruits For Glowing Skin: Know Their Skin Whitening Benefits

What Is The Cost Of Skin Whitening Treatment In Chennai?

Tan Removal Treatment: Cost, Procedure, Results & Prevention Tips



